what happens to a person long term with spin stimulator
Introduction
Nearly 800,000 people endure from traumatic spinal cord injury (SCI) worldwide every twelvemonth (Kumar et al., 2018). While some recovery is expected afterwards astute SCI, chronic SCI carries a stable prognosis with low probability of recovery. After the first yr of injury, less than two% of patients with motor complete spinal injury volition get incomplete by the 5th year after injury (Kirshblum et al., 2004). Interventions for chronic complete SCI generally focus on the medical direction of SCI-related complications, therapies to prevent musculoskeletal deterioration, and to provide adaptive strategies. Currently, predictors of neurological recovery in the acute phase include initial neurological status, incomplete injuries, and presence of a zone of partial preservation on imaging studies in complete injuries (Wilson et al., 2012). Only intensive neurorehabilitative therapies, such equally body weight-supported treadmill grooming, have level 3 evidence for improving functional ambulation in chronic SCI, and this likelihood is greater in motor incomplete injuries (Lam et al., 2007).
Epidural spinal cord stimulation (eSCS, SCS, or estim) has long been used for the treatment of chronic pain (Shealy et al., 1967) and is originally based on Melzack and Wall's (1965) gate control theory for peripheral neuromodulation of pain perception. As a neuromodulation platform capable of stimulating the central and peripheral nervous system, the therapeutic application of eSCS has been attempted on multiple fronts including Parkinson's affliction, MS, and SCI (Illis et al., 1980; Barolat et al., 1995; de Andrade et al., 2016). Several small clinical reports of spinal cord stimulation after chronic SCI have documented a promising potential to restore volitional movement in an immediate and long-term fashion (Angeli et al., 2018; Gill et al., 2018; Wagner et al., 2018). Withal, these improvements are accomplished just inside the context of intensive locomotor grooming coupled with eSCS.
The mechanisms by which electrical stimulation restores supraspinal control or modulates the office of the spinal cord remain unclear. Careful electrophysiology during motor-control tasks has revealed subtle supraspinal command in more than 80% of participants with clinically motor-consummate injuries (Sherwood et al., 1992), which indicates the presence of clinically silent supraspinal tracts potentially amenable to electrical stimulation. Acutely, epidural electrical stimulation primarily activates monosynaptic reflexes and generates complex outburst-like patterns initiated in the dorsal roots, identified as short-latency chemical compound muscle action potentials (Minassian et al., 2004). At a minimum, by activating collateral dorsal root sensory projections, stimulation may modulate the excitability of local circuitry to allow for diminished and quiescent supraspinal activity to exert greater influence.
While the potential biological effects of chronic eSCS on the office of injured spinal cords remain unexplored, a few reports exist that highlight the possibility of the evolution of restored volitional movement even subsequently eSCS is fabricated inactive, normally afterward months of intensive rehabilitation and stimulation. The progressive development of improved office due to chronic neuromodulation would provide a potentially impactful therapeutic platform. Notwithstanding, it has not been reported without intensive rehabilitation, which requires significant additional cost and dedicated time (French et al., 2007).
The Epidural Stimulation After Neurologic Damage (ESTAND) trial tests the effect of eSCS subsequently motor-consummate thoracic SCI on volitional movement and autonomic role without implementing locomotor therapy (Darrow et al., 2019). After several participants unexpectedly began to exhibit clinical prove of volitional motility without active eSCS, results were analyzed to further characterize this phenomenon. Here, we nowadays preliminary analysis of the offset seven patients in the trial across clinical observation, electrophysiology, and a functional bicycling task to characterize and compare those who did and did non develop volitional motility during periods without stimulation.
Methods
Subject Description
All the procedures described in this written report were canonical by the Hennepin Healthcare Inquiry Institute Institutional Review Board and with an Investigational Device Exemption from the United States Food and Drug Administration. Patients with chronic, traumatic SCI (more than 1 twelvemonth since injury) were recruited if they met the following criteria: older than 22 years of age, ASIA Impairment Scale (AIS) nomenclature A or B with a neurological level of injury between C6 and T10, full arm and hand strength and intact segmental reflexes below the level of injury. Participants were excluded if they had medical or psychological comorbidities that would significantly increase the adventure of surgery, astringent dysautonomia (systolic claret pressure fluctuation below l or in a higher place 200 mmHg) during autonomic testing, contractures, pressure level ulcers, recurrent urinary tract infection, unhealed spinal fracture, recent botulinum toxin use, or pregnancy. Once enrolled, subjects were asked to append any medications used for spasticity, for example, baclofen and oxybutynin. This assay includes seven participants that have completed lxxx% or more of the report. The six participants that have completed the study in its entirety were enrolled for a range of 1.26–1.47 years. Overall, participants had a mean age (±SD) of 42 ± 11.iv years, and a mean fourth dimension since injury (±SD) of 7.vii ± 4.8 years ranging from 3 to 17 years (Table 1). Three of the participants were female, and 4 were male. Utilizing the International Standards for Neurological Classification of Spinal String Injury (ISNCSCI) (Kirshblum et al., 2011), six participants were classified every bit AIS A, motor and sensory complete, and 1 subject was classified as AIS B, motor complete, and sensory incomplete. Subclinical motor complete injuries were further confirmed to exist electrophysiologically consummate with a baseline Brain Motor Control Assessment (BMCA) (Sherwood et al., 1996). All injuries were in the thoracic spine, with two participants at the T4 level, three participants at the T5 level, and two participants at the T8 level. Mechanisms of injury included falls, sports injuries, and motor vehicle accidents (MVA) (Tabular array one). Modified Ashworth Scale (MAS) (Meseguer-Henarejos et al., 2018) scores were nerveless at baseline before continuous stimulation therapy. A score of 0 to 4 was assigned to 4 muscle groups of each leg: hamstrings, quadriceps, gastrocnemius, and soleus. These scores were and so averaged for a mean lower extremity MAS score. All subjects included in this manuscript completed all 13 follow-up visits, for approximately 1 year, except for subject 7, who had completed 8 follow-upwards visits or approximately 9 months in the study.

Tabular array i. Demographic data: *ages are rounded to the closest decade.
Imaging
All participants provided thoracic spinal cord magnetic resonance imaging (MRI) at screening (Effigy one). Post-traumatic spinal cord changes observed on MRI included dorsal tethering, myelomalacia, syrinx, and cystic changes. In order to further categorize SCI severity and cloudburst, spinal cord anteroposterior (AP) and transverse diameters were manually measured at C7–T1 (above the injury) and at T9 (below the injury) to assess for spinal cord cloudburst (Freund et al., 2010). Both measures were matched to same-level normalized spinal string diameters from healthy participants (Frostell et al., 2016) and their differences computed for statistical modeling. One subject's MRI (Subject vii) was excluded from statistical assay because information technology was obtained during the astute SCI menstruation.
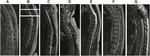
Figure 1. T2 sagittal thoracic magnetic resonance imaging (MRI) obtained prior to enrollment. (A) Subject field 1: spinal cord changes include dorsal tethering at T7 and syrinx from T8 to T10. (B) Subject two: syrinx at T7. Attributable to coronal scoliosis, a unmarried sagittal image did not provide a total view of the spinal culvert. A composite image of 3 different slices with the midline of the central canal kept as the centrality is shown. (C) Subject iii: T8 cord injury with cystic changes at the same level. (D) Discipline 4: T5 cord injury. (E) Subject 5: T5 cord injury and syrinx extending from T5 to T7. (F) Field of study six: T5 cord injury. (Thousand) Subject 7: T4 cord injury.
Implantation and Follow-Up
All participants were implanted with an epidural stimulator upon enrollment later on baseline information, including a detailed neurological test and cocky-reported questionnaires, were collected. Participants underwent epidural placement of a three-cavalcade, 16-contact paddle pb through a T11–T12 laminectomy and subcutaneous placement of a primary jail cell internal pulse generator (IPG) (Tripole and Proclaim Aristocracy, Abbott, Plano, TX, United States) in the lower lumbar area under general anesthesia (Figure ii). Intraoperative needle electromyogram (EMG) guided optimal paddle placement for symmetric and extended coverage of target spinal segments L2–S2. Starting 1 calendar month afterward implantation, 13 follow-up assessments, 30–45 days apart, were conducted involving stimulator setting reprogramming and study assessments. Participants were provided with a patient programmer and allowed to utilize specific stimulation settings for dissimilar goals such as volitional motility, spasticity control, core strength, and autonomic functions. Participants could utilise the stimulation throughout each day (upwards to 24 h a twenty-four hour period) after a calendar month of gradual adjustment to time and amplitude. A detailed clarification of report methods can exist found in a previous publication (Darrow et al., 2019). Observational data was nerveless using study personnel documentation from in-clinic follow-ups as well equally subject area self-reports.
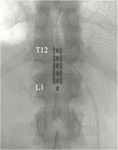
Figure 2. Placement of 5-6-5 epidural paddle lead through a T12 laminectomy, overlying the T12–L1 epidural space. Final pb placement is guided past intraoperative electromyogram (EMG).
Encephalon Motor Command Cess
The BMCA was conducted during subject screening and twice at each follow-up visit, without and with eSCS. The eSCS program was selected based on participants' preferences during the previous month of eSCS employ, likewise as objective data on the current calendar month's settings (Darrow et al., 2019). The BMCA is a neurophysiological cess of voluntary motor function using surface EMG over a series of three phases in the supine position: relaxation, reinforcement maneuvers, and voluntary movements (Sherwood et al., 1996). At the start of the trial, participants are instructed to follow a two-toned auditory cue equally the mark for the beginning and finish of each job. The reinforcement maneuvers include deep breath, neck flexion, Jendrassik maneuver, and bilateral shoulder shrug. The first fix of voluntary movements includes hip and knee flexion/extension with both legs, and so isolated left and right sides. The second ready of voluntary movements are ankle dorsiflexion and plantar flexion bilaterally, and the isolated left and correct sides. The participants are asked to endeavor the movements even if they are unable to practise and then and even if the requested movements are not produced. Surface EMG is measured through 15 pairs of surface electrodes on the following muscles bilaterally: paraspinal, iliopsoas, rectus femoris, tibialis anterior, extensor hallucis longus, gastrocnemius, rectus abdominis, and intercostals. A Nicollet EDX, ECR-xvi, research EMG is used at a sampling rate of 600 Hz.
Electromyogram was pre-processed by removing 60-Hz noise with a Fourier filter and then power calculated in windows of fourth dimension by boilerplate root hateful square (RMS) (Darrow et al., 2019). Command start and cease times were marked with labeled outcome timestamps in the EMG conquering system. Baseline fourth dimension windows for each trial started 3 s before the auditory cue and concluded 1 s before the auditory cue for all six volitional tasks. An auditory cue was manually synchronized with the event timestamp. Muscle activity ability during volitional task fourth dimension windows was averaged across musculus groups and across all tasks. Similarly, musculus activity voltage at residue before each job was averaged. The ratio of boilerplate muscle power during volitional tasks to baseline preceding the command to start volitional movement was used to measure out strength of volitional command and is represented in decibels [dB; , where P 5 is the power during volitional control, and P b is the power in the immediately preceding window to the command to move] and volition be referred to further on in this paper as volitional power.
Stationary Bike
The Muvi 300 cycle from MOTOmed was used to assess functional motion capabilities. The Muvi 300 includes a motor-assisted setting that facilitates training with minimal musculus forcefulness past switching from passive to active training without strain. The active assist motor can vary in speed (rpm) and resistance. When the strength sensitivity threshold is met, the motor ceases and the patients pedal on their own in the bike'south agile mode. The bike collects trial duration [seconds (s)], active and passive way duration (s), active and passive distance traveled [meters (m)], active and passive speed [rotations per minute (rpm)], work done in agile fashion [kilojoules (kj)], and average and maximum energy produced during active phase [watts (West)].
The factorial design bicycling task was added later on initial participants demonstrated enough move capabilities with active eSCS to allow for more robust functional assessments during follow-up sessions. Therefore, the data capture window for each participant differs based on the relative enrollment date. If the bike was implemented after a bailiwick initiated eSCS therapy, they did non undergo a baseline visit.
At the baseline visit, participants completed two motor-assisted cycle trials: one passive trial where the field of study was asked to relax and ane active where the field of study was asked to attempt to pedal. At each subsequent visit, the subject completed the aforementioned passive and active trials without stimulation and additional passive and active trials with selected preferred stimulation setting on. The duration of each trial was 2 min. As a result, in that location were two weather condition without stimulation analyzed: (1) no effort, no stimulation and (2) maximal try, no stimulation.
Statistical Analysis
RStudio (2015) software was used for statistical analyses. Isle of mann–Whitney U tests were used as non-parametric testing of differences between Spontaneous Volitional Move (SVM) group and non-Spontaneous Volitional Movement (not-SVM) grouping. Generalized linear models were used to assess linear relationships for BMCA volitional ability without stimulation during each study visit. Fixed effects tested to fit the model included: subjects, follow-up visit (time), Modified Ashworth scores earlier and after the report visit and volitional power with the stimulation on. A second model was used to assess the furnishings of spinal cord atrophy on average BMCA volitional power. Fixed effects tested to fit the model included: transverse and anteroposterior spinal cord diameter changes above and below the injury. When testing models with the same response variable, the best-fit model was called based on the lowest Akaike information benchmark (AIC). Biking data included an backlog of nada counts and overdispersed counts. It was therefore analyzed with a zero-inflated negative binomial regression that was found to be significantly superior to a negative binomial generalized linear model with the Vuong Non-nested Hypothesis exam (p < 0.005). For all statistical analyses, a p-value of less than 0.05 was considered to exist statistically significant. p-values are summarized in figures as one star (∗) for p < 0.05, 2 stars (∗∗) for p < 0.01, three stars (∗∗∗) for p < 0.001, and iv stars (****) for p < 0.0001.
Results
Study Population
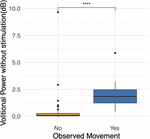
Four participants unexpectedly developed sustained volitional movement with stimulation turned off, referred to as the SVM group. In gild to ascertain grouping differences, participants from the SVM group were compared to the participants who only demonstrated movement with stimulation, referred to equally the non-SVM grouping (n = three) (Supplementary Table S1). Age (p = 0.285) and years post-injury (p = 0.476) were non significantly dissimilar betwixt the SVM and non-SVM groups. The differences of anteroposterior (p = 0.114) and transverse (p = 0.212) spinal string diameters from normal above the injury were not significantly dissimilar between SVM and non-SVM groups. The differences of anteroposterior (p = 0.4) and transverse (p = 0.4) spinal cord diameters from normal below the injury were not significantly unlike between SVM and non-SVM groups. Baseline spasticity scores (MAS) prior to continuous stimulation therapy were significantly higher at baseline in the SVM group (mean 2.44 ± 1.12) than in the non-SVM group (hateful 0.17 ± 0.29; p = 0.048). This difference is depicted in Figure iii.
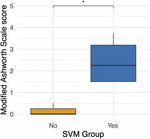
Effigy 3. Differences in baseline Modified Ashworth Scale scores between the spontaneous volitional motility (SVM) group (Yep) and not-SVM group (No). Participants in the SVM group had significantly college spasticity MAS scores than those in the non-SVM group. These differences were present before the offset of continuous eSCS therapy. *p < 0.05.
Across all participants, boilerplate daily stimulation use ranged from five to 21 h/solar day, with a mean of 13.7 ± v.eight h/day. Total eSCS fourth dimension at their final follow-upward visit ranged from 101.two to 454.five days, with a mean of 255.3 ± 115.3 days. There were no statistically significant differences in the total amount of stimulation used (p = 0.629) nor average daily stimulation (p = 1) between SVM and non-SVM groups. Within the SVM group, participants had undergone a range of 67.1–244.6 total days of stimulation at the time of their kickoff observed move, with a median of 87 days.
While this report does not involve an intensive rehabilitative therapy component, study personnel collected cocky-reported information in order to narrate modalities and hours of therapy undergone before and during the report. Six participants reported receiving rehabilitative therapy during acute care as well as full general and specialized in-patient rehabilitation. Prior to the study, 57% (4/7) of the participants reported receiving specialized SCI out-patient therapy, 29% (2/seven) reported receiving full general out-patient therapy, and 43% (iii/7) completed therapy at home. After implantation of the epidural stimulator, 43% (3/seven) of the participants reported receiving specialized SCI out-patient therapy, 14% (one/vii) reported receiving full general out-patient therapy, and 43% (three/7) completed therapy at domicile. Out of the vi participants who responded to retrospective surveys, 3 reported changing their exercise routine post-implantation.
Participants in the SVM group practiced a wide range of exercises spanning from range of motion, aerobic exercises, and general upper- and lower-body strength exercises up to specialized SCI out-patient therapy through adaptive gyms, clinic services with a concrete therapist, and fifty-fifty activity-based locomotor exercise programs for core strength, leg force, and balance. participants from the non-SVM group engaged in a like range of exercise modalities. Of note, 1 subject area in the non-SVM grouping participated in rehabilitative therapy that included using a standing frame, exoskeleton, and functional electric stimulation to aid with stretching sessions. Intensity of rehabilitation therapy varied throughout the overall bailiwick population (Table ii).

Table 2. Weekly practise schedule: reported exercises during report enrollment.
Observational Information
Volitional motion without epidural stimulation was observed amid the four participants (two females, ii males) in the SVM grouping equally early as iii months postal service-implantation. In three of the four participants, study personnel cited volitional movement without eSCS during the BMCA. A instance report form (CRF) was implemented to certificate and characterize observed movements with and without stimulation (Supplementary Video S1). Of those three participants, 100% demonstrated hip flexion and extension also every bit knee joint flexion without eSCS during at least one follow-upward visit. 2 out of the three exhibited knee extension as well as ankle dorsiflexion and plantar flexion during at least i follow-up visit (Figure 4A).
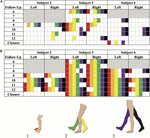
Effigy 4. Brain Motor Command Cess (BMCA) documented muscle activation without stimulation (A) and with stimulation (B) in the SVM group. Joint movements are color coded every bit follows: (1) red, ankle dorsiflexion and orange, ankle plantarflexion. (2) Yellow, knee flexion and dark-green, genu extension. (three) Purple, hip flexion and black, hip extension. Follow-upward visits 1–5 and Subject 5 are not included as the BMCA CRF had not been implemented nevertheless. Each discipline's introduction of the CRF class is color-coded by grayness boxes. Movements observed prior to the implementation of the BMCA CRF accept not been included here. Recorded movements only occurred during the volitional task windows of the BMCA (A). Recorded movements during BMCA in the absenteeism of stimulation. Field of study iii demonstrated persistent motility in the absenteeism of stimulation the earliest and across the virtually muscle groups among the SVM group. (B) Recorded movements during BMCA with stimulation on are included in order to exemplify how movement with stimulation is more prevalent before on in the study and across more musculus groups than move without stimulation.
Subject 5 provided a self-report noting right hip adduction, human knee flexion/extension, and plantar extension without eSCS at-abode during month xiii (Supplementary Video S2). Volitional motion without eSCS was not observed during their in-clinic BMCA testing.
By comparison, movement with stimulation (Figure 4B) was observed to be more consequent beyond musculus groups and more prominent in range of movement than without stimulation. When stimulation was on, all participants from both the SVM and non-SVM groups achieved volitional movement to varying degrees of magnitude and extent. These results are observed early on on in each subject field'southward study enrollment time, and they can be observed equally a directly effect of astute stimulation.
Brain Motor Command Cess
In society to characterize study observations, analysis of electrophysiological information from BMCA sessions without stimulation was performed. Sessions in which motility without stimulation was documented demonstrated increases in muscle activity specifically during volitional motor tasks compared to rest during baseline (Figure v). The magnitude of volitional motor control is represented by volitional power, as this ratio corrects for whatsoever involuntary muscle activity at baseline related to spasticity or spasms. In that location was a meaning divergence in volitional power, controlling for involuntary movement at rest, when motion was recorded in BMCA CRFs (p < 0.0005) (Effigy 6), meaning that clinical recognition of volitional motility is in understanding with EMG activity measured during the BMCA.
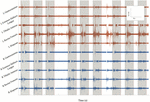
Effigy 5. BMCA at Subject area 2's follow-upwards visit thirteen: surface EMG electrical activeness recorded in volts over time for 8 bilateral lower extremity muscle groups. Orange traces include left muscle groups and blue traces include right musculus groups. Greyness boxes indicate volitional task events signaled by auditory cues. This subsample of EMG recording includes three cues for bilateral hip flexion, right hip flexion, and left hip flexion, respectively. EMG bursts can be seen to exist synchronized with the auditory cue followed by silent periods at rest, demonstrating volitional activity.
Effigy 6. Observations during BMCA. Differences in volitional power without stimulation (dB) when movement was observed and recorded on case report forms. High volitional power outliers when movement was non observed demonstrate the lower sensitivity of researcher observations. For reference, volitional power of iii dB represents an increase in muscle activity during volitional tasks of two times (200%) that of muscle activeness at baseline. Volitional ability of ten represents an increment in muscle action during volitional tasks of 10 times (grand%) that of the muscle activity at baseline. ***p < 0.001.
A generalized linear model was used to identify the effects of several variables on volitional power (dB) during volitional tasks without stimulation. Longitudinal explanatory variables tested included individual subject, follow-up visit, Modified Ashworth Scores during follow-up visits, and volitional power (dB) with stimulation on. When belongings all other variables constant, there was a significant but weak consequence of time (p = 0.016), a pregnant and strong positive consequence from Subject 3 (p = 0.0005) who demonstrated the nigh pregnant comeback, a meaning and moderate positive effect of spasticity earlier BMCA testing (p = 0.002) and a weak and trending toward significance positive effect of volitional power when stimulation was on (p = 0.066). To appraise for SCI differences, a generalized linear model for average volitional ability for each bailiwick was tested with the difference between transverse and anteroposterior spinal cord diameters to normal spinal cord measures above the injury (C7, T1) as explanatory variables; at that place were no pregnant effects. In a generalized linear model for average volitional ability for each subject with differences between transverse and anteroposterior spinal string diameters to normal spinal cord measures beneath the injury (T9) as explanatory variables, at that place was a negative strong effect of the AP spinal string diameter difference that trended toward significance (p = 0.071). In other words, greater anteroposterior spinal cord atrophy had a negative result on the corporeality of volitional power accomplished.
When assessing for the contained upshot of time, a trend is apparent as participants progress in follow-up visits (Figure seven), and the effect of fourth dimension is significant when correcting for other fixed effects, as is mentioned above. With the exception of Subject 3's early volitional activity at follow-up visit 4, the balance of the subject's volitional power emerges later on in the report. While Subject 5 is included in the SVM group due to observed volitional motion outside of written report assessments, these results are non reflected every bit volitional ability that is credible above baseline. On the other hand, while Subject 7 is included in the non-SVM grouping, they have not completed the report at the time of this manuscript submission, and the latest follow-upwardly visits demonstrate a rise in volitional power higher up baseline. To correct for intersubject and between-visit variability, pooling observations between SVM and not-SVM groups during three different stages of the study allows for a clearer estimation of the effect over time (Figure 8). Study periods were divided as follows: study period 1 corresponds to visits i–5 (approximately 5 months), written report catamenia 2 corresponds to visits 6–9 (approximately iv months), study menstruum corresponds to visits 10–13 (approximately 4 months). In report period 2, greater volitional ability in the SVM group than in the not-SVM group trends toward significance (p = 0.087), and in study period three, there is significantly greater volitional power in the SVM grouping (p < 0.001). Furthermore, only inside the SVM group is in that location a pregnant increase in volitional power from study period 1 to study menstruum 2 (p = 0.008) and from written report catamenia 2 to study menstruation three (p = 0.03).
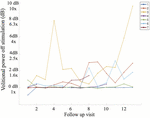
Figure vii. Boilerplate volitional power without stimulation (dB) at each written report follow-up visit including all seven participants. It is apparent that Subject 3 demonstrated volitional move the earliest in the study (follow-up visit 4) and with the greatest magnitude on sEMG, reaching volitional muscle activity 10 times greater (m%) than that at baseline on follow-up visit 13.
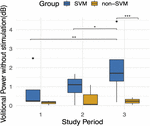
Effigy viii. Boilerplate volitional power without stimulation (dB) during three follow-up study periods. Written report flow 1 included follow-upwards visits ane–5. Study period ii included follow-up visits vi–9. Study period 3 included follow-up visits 10–13. An improvement over time is apparent simply in the SVM group and is statistically significant between study periods one and 3 (p = 0.008) and between study periods 2 and iii (p = 0.03). Volitional power between SVM and non-SVM groups is significantly greater in study period three (p < 0.001). *p < 0.05, **p < 0.01, ***p < 0.001.
Bike
Stationary bicycle trials proved complementary to electrophysiological measures as a functional assessment. During i participant's best bike trial, Subject field iii (Supplementary Video S3), they exerted 235 J of work that amounted to agile pedaling without motor assistance for 94.two% of the trial fourth dimension and 96.four% of the altitude traveled. These results occurred after 6 months of chronic eSCS. In the SVM group, the primeval activity emerged at follow-upward visit 6 and the latest at follow-upwardly visit 10.
A zero-inflated negative binomial regression for two selected dependent variables, distance traveled and work, was constructed including the post-obit independent variables: individual participants and the interaction between groups (SVM vs. non-SVM) and pedaling endeavour provided. When belongings all other variables abiding, at that place was a significantly strong positive effect on distance traveled (p < 0.005) and piece of work (p < 0.005) only when participants from the SVM grouping attempted to pedal (Figure nine).

Figure ix. Functional movement without stimulation: graphs demonstrate differences betwixt Groups (0: non-SVM group and 1: SVM group) and between volitional effort (n = no endeavour, y = with endeavour). Means are symbolized by blackness points. (A) Altitude traveled without help of the bicycle motor is plotted, when correcting for zero inflated data. There is a significant positive upshot on distance when participants from the SVM group attempted to pedal. (B) Pedaling work exerted is plotted, when correcting for zero inflated data. Participants in the SVM grouping significantly accomplished greater piece of work when they provided endeavour compared to the not-SVM grouping. *** refers to a p-value of <0.001.
Discussion
Preliminary data from the ESTAND trial suggests that long-term or chronic eSCS tin induce plastic changes in chronic, severely injured spinal cords through restored volitional movement without stimulation and without significant intensive rehabilitation. More than one-half of the first vii patients were observed to exhibit volitional movement without stimulation, which agreed with the more sensitive electrophysiology, and resulted in marked improvements in a functional cycling task.
None of the participants included in this cohort demonstrated traditional signs of discomplete spinal cord injuries earlier eSCS therapy began (Sherwood et al., 1992). Despite the fact that all participants exhibited motor-complete traumatic spinal string injuries confirmed with MRI, clinical testing, and electrophysiological testing at screening, more than than half of the participants demonstrated the reported improvements in volitional movement capabilities with stimulation inactive. It is important to emphasize that before these improvements were apparent, there was no indication that study participants had different responses to long-term eSCS because, grossly, all participants demonstrated improvements in volitional muscle activity when epidural stimulation was active. When comparing researcher-observed movements during BMCA off and on stimulation, active stimulation allowed for volitional joint movements that spanned beyond more muscle groups and more consistently beyond study visits. However, joint movements observed off stimulation represented a subset of those facilitated by eSCS at similar fourth dimension points. This finding might indicate that some of the same circuits that are potentiated past active stimulation are those responsive to chronic eSCS-facilitated plasticity.
Predictors of Recovery
In an effort to characterize each subject's propensity for this blazon of recovery, several descriptive field of study characteristics were included such as fourth dimension since injury, mechanism of injury, injury level, vertebral fracture level, fusion level, and imaging studies. None of these factors were meaning in determining if a subject area would develop movement without stimulation. Overall, the heterogeneity between participants exemplifies how within the about severe subgroup of the AIS scale, there are no current adequate measures to narrate the functional capacity of the spinal cord. Adequate measures that reflect the caste of preserved and quiescent supraspinal tracts across the spinal cord lesion could allow for phenotyping those responsive to neuromodulation.
In our cohort, spasticity scores before initiating eSCS therapy were slightly greater in the SVM group than in the not-SVM group, which was statistically significant. Moreover, longitudinal spasticity scores before each BMCA session had a meaning positive effect on volitional power. When assessing differences among motor-complete SCI participants, Sangari et al. (2019), compared spastic and not-spastic subgroups and reported that motor evoked potentials (MEP) were merely nowadays in the spastic subgroup, suggesting that spasticity might be a marker for preserved corticospinal tract axons (Sangari et al., 2019). Greater spasticity at baseline and during chronic eSCS therapy might reflect preserved but silent corticospinal tracts that served as substrates for the plastic effects of eSCS neuromodulation in the SVM grouping or may highlight the part that higher baseline spasticity may play in electrophysiology. Furthermore, high spasticity phenotypes might exist more than susceptible to the immediate depolarizing effects and long-term neuromodulation effects of eSCS that restore inhibition of uncontrolled spinal string excitability and potentiate functional activation of the spinal string (D'Amico et al., 2014). Although the mechanisms might be unclear, spasticity should be further assessed as a predictor and biomarker for eSCS-mediated spontaneous recovery.
Measures of spinal cord atrophy were also assessed as a marker of injury severity. Only anteroposterior atrophy below the level of injury was plant to have a strong issue on volitional power that did not quite see our criteria for significance. In chronic motor complete spinal string injuries, Sangari et al. (2019), reported MEP size to be positively correlated with the degree of spared tissue in lateral regions of the spinal cord above the injury. Our results should exist interpreted with circumspection as spinal cord diameter changes have been reported as a mensurate of SCI severity only higher up the level of injury (Freund et al., 2010; Sangari et al., 2019), and the measurements in this cohort were express by suboptimal MRI studies due to hardware artifacts and variability in SCI chronicity. As a result, imaging metrics did non prove to be useful predictors of recovery into the SVM group.
Most importantly, despite the large number of standard characteristics used to draw SCI, all only spasticity proved to be ineffective at predicting the variability in the development of motion without stimulation despite a uniform comeback with agile stimulation. In other words, at that place are no major predictors of the development of movement without stimulation identified thus far that would restrict the potential future use of eSCS as a therapy.
Function of Concurrent Rehabilitation in eSCS Recovery
Since the kickoff case of eSCS in SCI aiming to restore volitional movement was reported in 2004 (Carhart et al., 2004), there have been reports of three chronic SCI patients with eSCS therapy who have regained some level of volitional movement in the absenteeism of stimulation after intensive locomotor therapy. Angeli et al. (2014), reported motion without eSCS in i 32-yr-old male person patient with an AIS B injury subsequently 38 weeks of intensive locomotor training that included 80 sessions of full weight-begetting stand training and 80 sessions of step training with body weight support equally well as habitation-based volitional training (Rejc et al., 2017). Wagner et al. (2018), also reported two participants, a 28-yr-old male person with AIS C injury and a 35-year-old male with AIS D injury both enrolled half dozen years after injury, who demonstrated improvements in walking indices and motor scores without eSCS after participating in 5 months post-implantation of overground and treadmill locomotor training using a gravity assist device iv to five times a calendar week. In contrast, the ESTAND report did not prescribe the utilise of intensive locomotor training. In addition to their baseline rehabilitation therapies (described in Table ii), participation in this study involved a minimum of x min of daily visually cued flexion–extension tasks at home, ninety min of supine flexion–extension tasks during monthly BMCA, and x min of motor-assisted pedaling during monthly bike testing. Participants utilized eSCS in their daily activities according to their needs and preferences upward to 24 h per day, and more contained rehabilitation therapy was non associated with any clear do good.
Despite heterogeneity in daily time and endeavor defended to physical therapy among all participants, more than one-half of the participants (SVM grouping) demonstrated progressive statistically significant improvements in volitional move in the off stimulation state during the report period. As a consequence, in that location were statistically significant improvements in the ability to cycle without assistance, providing the footing for cost-effective domicile-based therapies to provide incremental improvements in muscle mass, cardio-metabolic risk factors and activities of daily living equally well every bit a platform for activity-dependent plasticity (Shen et al., 2018; Gorgey et al., 2019).
While activity-based plasticity is frequently associated with rehabilitation therapies, in that location is a possibility that directly increasing volitional move, increased use, and reliance on these improvements may facilitate more subtle and chronic activity-based plasticity in the sense that directed motor control during normal everyday life drives plastic changes. However, more intensive concurrent rehabilitation outside of the study did not bulldoze further recovery, which is exemplified by the fact that the participant who underwent the about all-encompassing specialized SCI therapy earlier and during the study did non develop spontaneous move without stimulation. To our cognition, this is the first study of eSCS-induced plasticity of volitional motility in the absenteeism of concurrent prescribed intensive locomotor grooming therapy after motor-complete SCI. While the Eastward-Stand up trial remains generalizable past allowing for a wide range of independent therapy, careful information collection of previous therapy regimens may prove useful for assessing further contribution through modeling.
Limitations and Future Directions
I limitation in this study relates to an undefined off-stimulation time period. As our protocol allows participants to apply as much stimulation as they require for their daily activities and comfort. Every bit such, there was no established eSCS-weaning time across a minimum of two h when testing for off-stimulation activeness. An important confounder to consider is whether the reported results might exist related to stimulation carry-over effect, described as temporarily persisting changes in spinal cord circuit excitability. This has been described clinically in relation to spasticity modulation in SCI patients as lasting from hours to days (Cook, 1976; Dimitrijevic Yard. G. et al., 1986; Dimitrijevic M. R. et al., 1986; Barolat et al., 1988). Whether the effects in the absence of eSCS in the participants in this report are temporary or persistent over longer periods of fourth dimension with stimulation off will take to be farther assessed.
In this manuscript, the observed movements during the BMCA were non matched to the intended volitional task. Instead, pooling of observed movements was compared to pooled EMG musculus activation during all volitional tasks. In the time to come, a thorough assay of the accuracy of muscle activation for each intended joint movement should be performed. The preliminary results at this stage were not sufficiently powered to assess these outcomes. Hither we demonstrate the robust evidence of muscle activeness magnitude. Muscle action accurateness will accept to be assessed in time to come larger studies.
Although this is the largest group reported of SCI patients treated with eSCS to restore volitional movement, results should be interpreted with circumspection due to the small number of participants. In the hereafter, studies with larger cohorts might allow for adequate eSCS therapy phenotyping. In-depth analysis of stimulation usage might point to a dose–response human relationship that was not credible in this report. Furthermore, neurophysiological testing such as somatosensory evoked potentials, electroencephalogram, and transcranial magnetic stimulation motor-evoked potentials might permit for categorizing the furnishings of chronic eSCS on ascending pathways, cortical representation, and descending pathways, respectively. Correlating these results with high-resolution MRI at enrollment to find spinal cord expanse differences might aid in further characterizing the heterogeneity of spinal string injuries and identifying the degree of preserved pathways that may serve as substrates for recovery (Freund et al., 2010). With these results, nosotros hope to farther evidence the role of eSCS in SCI rehabilitation and exemplify how the furnishings of chronic eSCS are only starting to be credible.
Data Availability Statement
The datasets presented in this commodity are non readily available because the ESTAND trial is currently ongoing and datasets retain some identifiable information. A express dataset may be made bachelor. Requests to access the datasets should be directed to David P. Darrow, darro015@umn.edu.
Ethics Argument
The studies involving human participants were reviewed and approved by the Human Subjects Inquiry Committee, Hennepin Healthcare Organisation. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individuals for the publication of whatever potentially identifiable images or data included in this article.
Author Contributions
DD provided all oversight for the study. DD, APa, AK, APh, U.s., and TN designed the study. DD, DF, APa, and US performed the critical surgical procedures of this study. CH, AA, SV, DS, DB, and DF performed the study procedures and information drove. DD, TN, NP, and IP analyzed the information. IP wrote the manuscript with support from DD, CH, AA, SV, and DS. All authors provided critical feedback and helped shape the research, analysis, and manuscript, and approved the final manuscript.
Funding
This written report was funded by a MN State SCI/TBI grant from the Minnesota Function of Higher Didactics (grant number 159800).
Disharmonize of Interest
US reports having no conflicts of interest relevant to this article. DD, AP, and TN study having several patents related to neuromodulation and are cofounders of a neuromodulation company.
The remaining authors declare that the enquiry was conducted in the absenteeism of any commercial or fiscal relationships that could exist construed as a potential conflict of interest.
Acknowledgments
We would similar to give thanks the Minnesota Office of College Education SCI/TBI Grant Program (grant number 159800) for the funding to behave out this study and St. Jude/Abbott for a generous device donation.
Supplementary Textile
The Supplementary Material for this article can be found online at: https://world wide web.frontiersin.org/articles/10.3389/fnsys.2020.00035/total#supplementary-textile
TABLE S1 | Summary of means and standard deviations (SD) for all variables used to appraise differences betwixt groups. P-values are obtained from single variable Mann-Whitney U tests. SVM: Spontaneous volitional move. Non-SVM: No spontaneous volitional movement.
VIDEO S1 | BMCA video recording of subject 3 with stimulation off. Subjects lie supine with surface electromyography electrodes placed while they perform volitional tasks triggered by auditory cues. At follow up 6, the subject achieves bilateral simply predominantly left hip internal rotation and flexion as well as left dorsiflexion of the toes. At follow up nine, he again achieves left hip internal rotation and flexion as well as left dorsiflexion of the toes.
VIDEO S2 | Dwelling house video recording of subject iv demonstrates volitional right knee joint extension in the absence of stimulation.
VIDEO S3 | Stationary bike video recording of subject 3 at Follow-up visit six. This trial involves 2 min when subjects intend to pedal with stimulation off. In this item trial shown, the discipline achieved active pedaling for 113 seconds (94% of trial fourth dimension) for a distance of 268 meters (96% of trial distance).
Abbreviations
BMCA, Brain Motor Control Assessment; eSCS, epidural spinal cord stimulation; ESTAND, Epidural Stimulation Afterward Neurologic Damage; MAS, Modified Ashworth Scale; SVM, Spontaneous Volitional Movement.
References
Angeli, C. A., Boakye, M., Morton, R. A., Vogt, J., Benton, Grand., Chen, Y., et al. (2018). Recovery of over-ground walking after chronic motor complete spinal string injury. North. Engl. J. Med. 379, 1244–1250. doi: 10.1056/NEJMoa1803588
PubMed Abstract | CrossRef Full Text | Google Scholar
Angeli, C. A., Edgerton, 5. R., Gerasimenko, Y. P., and Harkema, Due south. J. (2014). Altering spinal cord excitability enables voluntary movements after chronic consummate paralysis in humans. Brain J. Neurol. 137(Pt 5), 1394–1409. doi: x.1093/encephalon/awu038
PubMed Abstruse | CrossRef Total Text | Google Scholar
Barolat, M., Myklebust, J. B., and Wenninger, W. (1988). Effects of spinal cord stimulation on spasticity and spasms secondary to myelopathy. Appl. Neurophysiol. 51, 29–44. doi: x.1159/000099381
PubMed Abstract | CrossRef Full Text | Google Scholar
Barolat, G., Singh-Sahni, K., Staas, W. E. Jr., Shatin, D., Ketcik, B., and Allen, K. (1995). Epidural spinal cord stimulation in the management of spasms in spinal cord injury: a prospective report. Stereotactic Funct. Neurosurg. 64, 153–164.
Google Scholar
Carhart, M. R., He, J., Herman, R., D'Luzansky, Southward., and Willis, Due west. T. (2004). Epidural spinal-cord stimulation facilitates recovery of functional walking following incomplete spinal-cord injury. IEEE Trans. Neural Syst. Rehabil. Eng. 12, 32–42. doi: 10.1109/TNSRE.2003.822763
PubMed Abstract | CrossRef Full Text | Google Scholar
D'Amico, J. M., Condliffe, E. G., Martins, Thou. J. B., Bennett, D. J., and Gorassini, G. A. (2014). Recovery of neuronal and network excitability later spinal cord injury and implications for spasticity. Front end. Integr. Neurosci. 8:36. doi: x.3389/fnint.2014.00036
PubMed Abstract | CrossRef Full Text | Google Scholar
Darrow, D., Balser, D., Netoff, T. I., Krassioukov, A., Phillips, A., Parr, A., et al. (2019). Epidural spinal string stimulation facilitates immediate restoration of dormant motor and autonomic supraspinal pathways after chronic neurologically complete spinal string injury. J. Neurotrauma 36, 2325–2336. doi: 10.1089/neu.2018.6006
PubMed Abstract | CrossRef Full Text | Google Scholar
de Andrade, E. Chiliad., Ghilardi, M. G., Cury, R. G., Barbosa, Eastward. R., Fuentes, R., Teixeira, M. J., et al. (2016). Spinal cord stimulation for Parkinson's disease: a systematic review. Neurosurg. Rev. 39, 27–35.
Google Scholar
Dimitrijevic, Grand. M., Dimitrijevic, 1000. R., Illis, L. Due south., Nakajima, One thousand., Sharkey, P. C., and Sherwood, A. M. (1986). Spinal cord stimulation for the control of spasticity in patients with chronic spinal cord injury: I. Clinical observations. Centr. Nerv. Syst. Trauma iii, 129–144. doi: 10.1089/cns.1986.three.129
PubMed Abstract | CrossRef Full Text | Google Scholar
Dimitrijevic, 1000. R., Illis, 50. S., Nakajima, K., Sharkey, P. C., and Sherwood, A. M. (1986). Spinal cord stimulation for the command of spasticity in patients with chronic spinal cord injury: II. Neurophysiologic observations. Centr. Nerv. Syst. Trauma 3, 145–152. doi: x.1089/cns.1986.3.145
PubMed Abstract | CrossRef Total Text | Google Scholar
French, D. D., Campbell, R. R., Sabharwal, Due south., Nelson, A. L., Palacios, P. A., and Gavin-Dreschnack, D. (2007). Health care costs for patients with chronic spinal cord injury in the Veterans Health Administration. J. Spinal Cord Med. 30, 477–481. doi: x.1080/10790268.2007.11754581
PubMed Abstruse | CrossRef Full Text | Google Scholar
Freund, P. A. B., Dalton, C., Wheeler-Kingshott, C. A. M., Glensman, J., Bradbury, D., Thompson, A. J., et al. (2010). Method for simultaneous voxel-based morphometry of the brain and cervical spinal cord area measurements using 3D-MDEFT. J. Magn. Reson. Imaging JMRI 32, 1242–1247.
Google Scholar
Frostell, A., Hakim, R., Thelin, Eastward. P., Mattsson, P., and Svensson, Yard. (2016). A review of the segmental diameter of the healthy human spinal cord. Front. Neurol. 7:238. doi: 10.3389/fneur.2016.00238
PubMed Abstract | CrossRef Full Text | Google Scholar
Gill, M. L., Grahn, P. J., Calvert, J. S., Linde, K. B., Lavrov, I. A., Strommen, J. A., et al. (2018). Neuromodulation of lumbosacral spinal networks enables independent stepping later complete paraplegia. Nat. Med. 24, 1677–1682. doi: 10.1038/s41591-018-0175-7
PubMed Abstract | CrossRef Full Text | Google Scholar
Gorgey, A. S., Khalil, R. E., Davis, J. C., Carter, W., Gill, R., Rivers, J., et al. (2019). Skeletal muscle hypertrophy and attenuation of cardio-metabolic adventure factors (SHARC) using functional electrical stimulation-lower extremity cycling in persons with spinal string injury: written report protocol for a randomized clinical trial. Trials 20:526. doi: 10.1186/s13063-019-3560-8
PubMed Abstract | CrossRef Total Text | Google Scholar
Illis, L. South., Sedgwick, E. 1000., and Tallis, R. C. (1980). Spinal cord stimulation in multiple sclerosis: clinical results. J. Neurol. Neurosurg. Psychiatry 43, 1–14.
Google Scholar
Kirshblum, Due south. C., Burns, S. P., Biering-Sorensen, F., Donovan, Westward., Graves, D. E., Jhan, A., Johansen, M., et al. (2011). International standards for neurological classification of spinal string injury. J. Spinal Cord Med. 34, 535–546. doi: 10.1179/204577211x13207446293695
PubMed Abstruse | CrossRef Full Text | Google Scholar
Kirshblum, South., Millis, S., McKinley, W., and Tulsky, D. (2004). Late neurologic recovery after traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 85, 1811–1817. doi: 10.1016/j.apmr.2004.03.015
PubMed Abstract | CrossRef Full Text | Google Scholar
Kumar, R., Lim, J., Mekary, R. A., Rattani, A., Dewan, M. C., Sharif, S. Y., et al. (2018). Traumatic spinal injury: global epidemiology and worldwide book. World Neurosurg. 113, e345–e363. doi: 10.1016/j.wneu.2018.02.033
PubMed Abstract | CrossRef Total Text | Google Scholar
Lam, T., Eng, J. J., Wolfe, D. Fifty., Hsieh, J. T., and Whittaker, M. and the Scire Research Team (2007). A systematic review of the efficacy of gait rehabilitation strategies for spinal cord injury. Top. Spinal Cord Injury Rehabil. thirteen, 32–57. doi: 10.1310/sci1301-32
PubMed Abstract | CrossRef Full Text | Google Scholar
Meseguer-Henarejos, A. B., Sanchez-Meca, J., Lopez-Pina, J. A., and Carles-Hernandez, R. (2018). Inter- and intra-rater reliability of the Modified Ashworth Scale: a systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 54, 576–590. doi: x.23736/S1973-9087.17.04796-7
PubMed Abstract | CrossRef Full Text | Google Scholar
Minassian, K., Jilge, B., Rattay, F., Pinter, Grand. M., Binder, H., Gerstenbrand, F., et al. (2004). Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar string: electromyographic study of chemical compound muscle action potentials. Spinal String 42, 401–416.
Google Scholar
Rejc, Due east., Angeli, C. A., Atkinson, D., and Harkema, S. J. (2017). Motor recovery later action-based preparation with spinal string epidural stimulation in a chronic motor complete paraplegic. Sci. Rep. 7:13476. doi: 10.1038/s41598-017-14003-w
PubMed Abstract | CrossRef Full Text | Google Scholar
RStudio (2015). RStudio: Integrated Development for R. Boston, MA: RStudio, Inc.
Google Scholar
Sangari, S., Lundell, H., Kirshblum, Due south., and Perez, M. A. (2019). Residual descending motor pathways influence spasticity after spinal cord injury. Ann. Neurol. 86, 28–41. doi: 10.1002/ana.25505
PubMed Abstract | CrossRef Full Text | Google Scholar
Shealy, C. N., Mortimer, J. T., and Reswick, J. B. (1967). Electrical inhibition of pain by stimulation of the dorsal columns: preliminary clinical written report. Anesth. Analg. 46, 489–491.
Google Scholar
Shen, C., Liu, F., Yao, Fifty., Li, Z., Qiu, Fifty., and Fang, S. (2018). Effects of MOTOmed movement therapy on the mobility and activities of daily living of stroke patients with hemiplegia: a systematic review and meta-analysis. Clin. Rehabil. 32, 1569–1580. doi: 10.1177/0269215518790782
PubMed Abstract | CrossRef Full Text | Google Scholar
Sherwood, A. M., Dimitrijevic, Thousand. R., and McKay, W. B. (1992). Evidence of subclinical brain influence in clinically complete spinal string injury: discomplete SCI. J. Neurol. Sci. 110, 90–98. doi: 10.1016/0022-510x(92)90014-c
CrossRef Total Text | Google Scholar
Sherwood, A. M., McKay, W. B., and Dimitrijević, Chiliad. R. (1996). Motor control after spinal string injury: assessment using surface EMG. Musculus Nerve xix, 966–979.
Google Scholar
Wagner, F. B., Mignardot, J.-B., Le Goff-Mignardot, C. M., Demesmaeker, R., Komi, S., Capogrosso, Thousand., et al. (2018). Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 563, 65–71. doi: x.1038/s41586-018-0649-2
PubMed Abstract | CrossRef Full Text | Google Scholar
Wilson, J. R., Cadotte, D. W., and Fehlings, M. Thou. (2012). Clinical predictors of neurological outcome, functional status, and survival after traumatic spinal cord injury: a systematic review. J. Neurosurg. 17(one Suppl.), xi–26. doi: 10.3171/2012.4.AOSPINE1245
PubMed Abstract | CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fnsys.2020.00035/full
0 Response to "what happens to a person long term with spin stimulator"
Post a Comment